Resources for Principal Investigators
- ClinicalTrials.gov Guidance for PIs
- ACRP Training
-
GW SMHS makes available online Association of Clinical Research Professionals (ACRP) clinical research training at no cost to GW and MFA participants to enhance the quality and safety of our clinical and translational research.
Complete Enhanced Training in Clinical Research: Take one course or complete the recommended training for GW and MFA clinical researchers
Courses are available to GW and MFA faculty and staff working on human subject studies. Courses are online and self-paced.Please note: If you have a personal ACRP account, do not follow the instructions below. Instead, Contact ACRP Customer Care with a request to be aligned to the Children’s National Hospital ACRP Learning Environment.
To Register for an ACRP account:- Visit the New User Link: You MUST use this link to login. This is the ONLY link that will allow you to create an ACRP account and align with your organization. Failing to use this link will require assistance from ACRP Customer Care.
- Enter your Organizational Email: Enter your GWU email address and click the “Search” button.
- Register with your Organization: When your organization appears, click the “Register with this Organization” button.
NOTE: This training is offered through a partnership with Children’s National Hospital so you will need to select them as your organization. - Enter Your Profile Information: Complete the account creation process by entering your demographic information
- Click the “My ACRP Learning Environment” Button: Click on the “My ACRP Learning Environment” button to be redirected to the ACRP Learning Environment, where you will access your courses. This is the link you will use to access the ACRP Learning Environment now that you have an account. Bookmark it!
- Look for the Logo: Once you are directed to your ACRP Learning Environment dashboard, make sure you are seeing the Children’s National logo in the upper left corner. If you do not see the logo, please Contact ACRP Customer Care.
You can find all these steps, plus additional resources on the linked pdf.
For questions, please contact us at clinicalresearch [at] mfa [dot] gwu [dot] edu (clinicalresearch[at]mfa[dot]gwu[dot]edu).
- Training is provided at no cost to our teams
- Recommended courses vary by role on study as coordinators, managers & PIs
- About 15 hours to complete
- CITI Training for PIs
-
All Principal Investigators must complete mandatory training through the CITI Program prior to engaging in any clinical research activity. It is vital to the integrity of research compliance that these courses are completed, and it is that person’s responsibility to not allow these courses to expire.
The George Washington University requires all researchers to take the following courses:
- Biomedical Investigators, expires every 2 years
- Good Clinical Practice (GCP), expires every 3 years
- Health Information Privacy and Security (HIPS), never expires
**The Refresher Course should only be taken if you have completed the Basic Course and it has expired**
- Conflict of Interest
-
Unsure if you have a conflict of interest? Check out the links below for more information.
Office of Research Integrity
Office of Ethics, Compliance, and Risk - New Research Position Packet
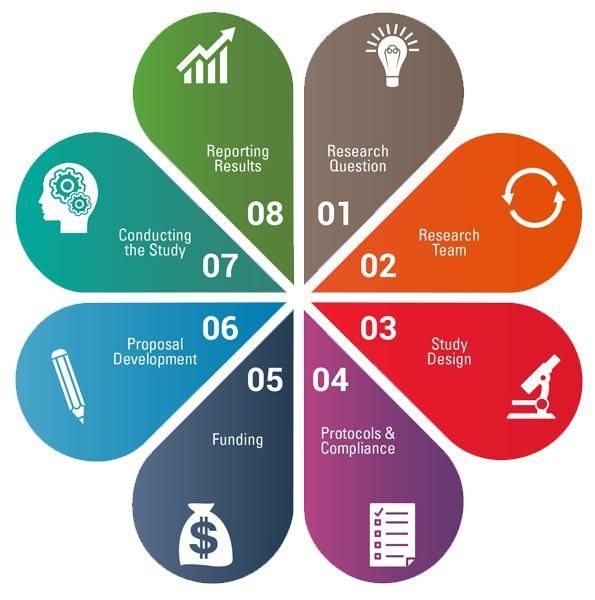
The CTSI-CN Virtual Organizer connects GW and CNH investigators, staff, and community partners with local research training, tools, and navigators. This web-based organizer integrates knowledge, resources, and best practices from the CTSI-CN and the CTSA network to assist investigators and research teams through the 8 steps of the research cycle.